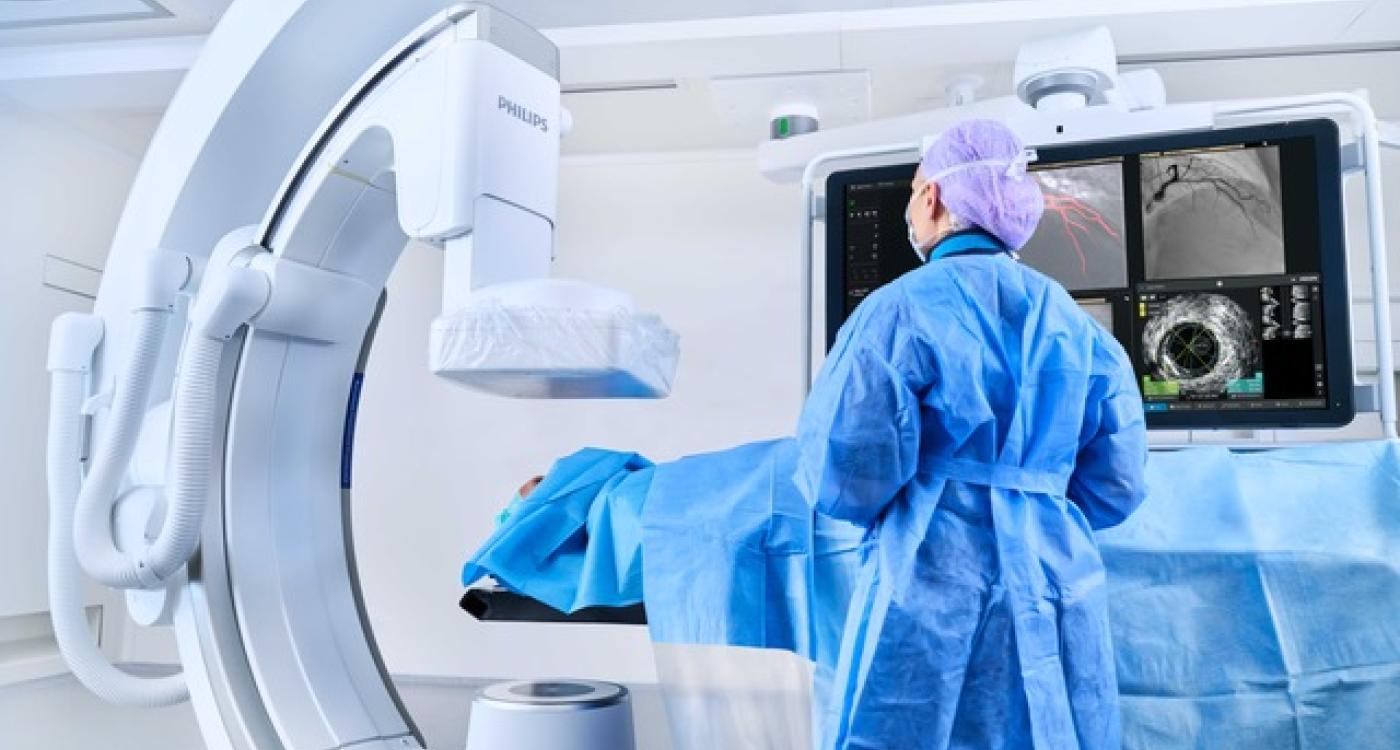
Royal Philips, a global leader in health technology, has initiated the RADIQAL (Radiation Dose and Image Quality Trial) study, a prospective, randomized, multi-center clinical investigation assessing the effectiveness of its new ultra-low X-ray dose technology in coronary interventions.
The trial will enroll 824 patients with coronary artery disease (CAD) across six hospitals in Spain, the Czech Republic, Denmark, and the United States. The first patient has already been enrolled at Aarhus University Hospital, Denmark.
“Minimizing radiation exposure without compromising procedural performance is essential in interventional cardiology,” said Dr. Javier Escaned, Principal Investigator and Professor of Cardiology at Hospital Clínico San Carlos, Madrid. “RADIQAL is designed to generate real-world evidence on whether Philips’ new technology can achieve this balance, even in complex clinical scenarios involving diluted contrast media.”
The study compares Philips’ new ultra-low dose protocol with the established ClarityIQ technology, both integrated into the company’s Azurion image-guided therapy system. The new protocol is designed to reduce radiation exposure by up to 50% compared to current low-dose settings.
Already CE-marked under the EU MDR framework, the technology is aimed at improving safety for both patients and clinical staff—especially relevant for individuals with high body mass index (BMI) or those requiring repeat interventions.
“One of the core innovation drivers in interventional cardiology is to reduce radiation exposure while preserving or enhancing image quality,” said Dr. Darshan Doshi, Head of Medical & Clinical at Philips Image-Guided Therapy Devices and Interventional Cardiologist at Massachusetts General Hospital, Boston. “High-quality, low-dose imaging supports confident decision-making and protects both patients and operators.”
The RADIQAL study will provide valuable insights into how advanced imaging protocols perform under real-world conditions and aligns with Philips’ broader mission to improve clinical outcomes through innovation in dose management and image-guided therapy.
About the RADIQAL Study
RADIQAL is a Philips-sponsored, prospective, randomized, unblinded clinical investigation evaluating the clinical performance of a new ultra-low dose imaging technology for coronary artery disease procedures. The trial focuses on radiation exposure, image quality, and procedural efficiency.
About Azurion
Philips Azurion is an advanced image-guided therapy system designed for cardiovascular interventions. It integrates real-time imaging, intuitive workflow, and smart software features to support precise, minimally invasive procedures.
About Philips in Interventional Cardiology
Philips offers a comprehensive portfolio for image-guided therapy, including angiography systems, intravascular imaging, physiology measurement, and integrated therapy devices. The company focuses on innovation that enhances outcomes, reduces risk, and simplifies complex procedures for clinicians worldwide.