Lunit has launched the latest version of its chest X-ray analysis platform, Lunit INSIGHT CXR4, now officially CE-certified under the EU Medical Device Regulation (MDR). The updated solution brings expanded AI capabilities to radiology departments across Europe.
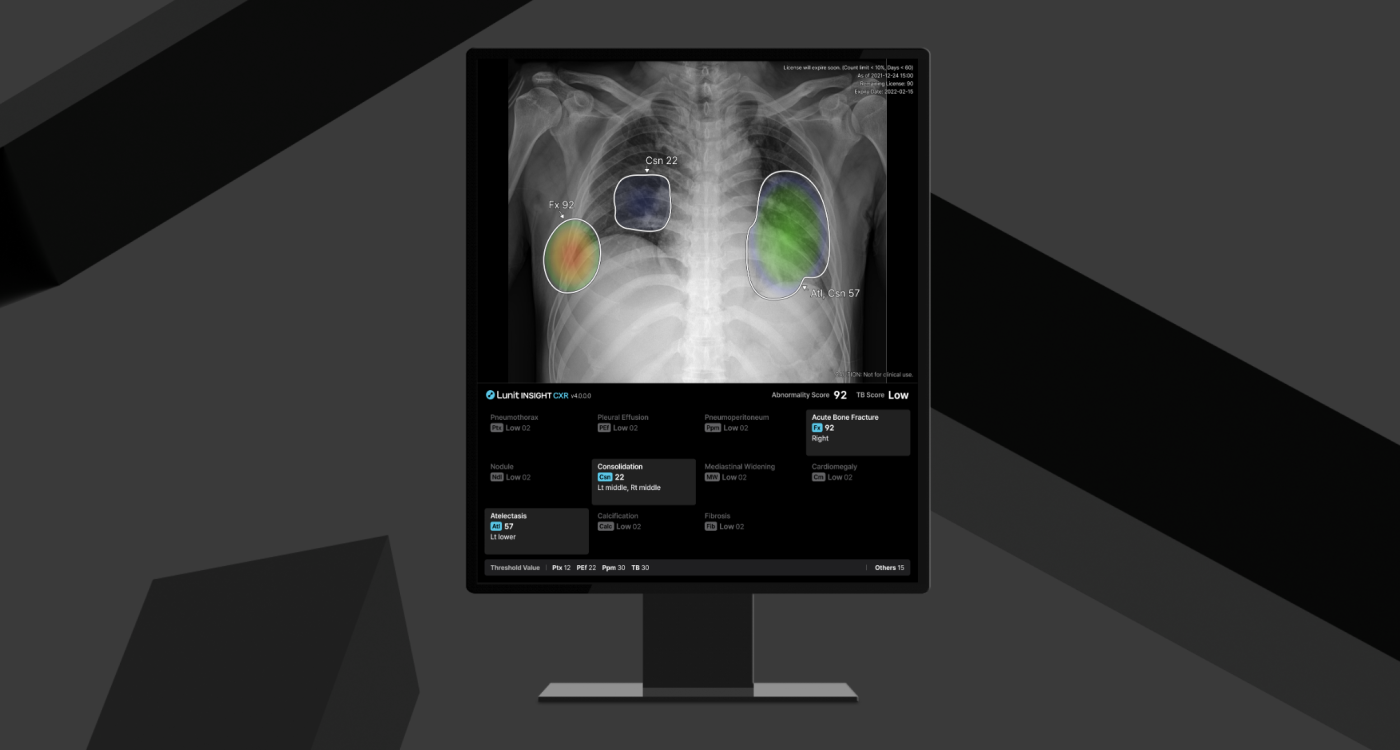
Trained on more than 1.2 million real-world chest X-rays, CXR4 detects 12 thoracic abnormalities—including lung nodules, pneumonia, and pneumothorax—and now adds acute bone fracture detection to its list of findings. The goal: support radiologists with more accurate, efficient diagnostics in high-throughput environments.
New features designed for clinical workflow
Alongside the expanded detection scope, CXR4 introduces several workflow-oriented features:
Normal FlaggingUnlike simple absence-of-finding filters, CXR4 applies a robust AI model to proactively identify truly normal studies with a 99.5% negative predictive value (NPV). For these low-risk cases, the system generates structured reports automatically, reducing reading time while maintaining diagnostic confidence.
Current-Prior ComparisonThe system enables side-by-side analysis of current and previous images to assess disease progression or resolution. Nodule comparison is also included—particularly valuable in lung cancer screening and follow-up contexts.
New Finding: Acute Bone FracturesSubtle post-traumatic fractures—often missed in busy clinical settings—are now detectable via CXR4, supporting improved triage and interpretation speed.
Market access and broader integration
With CE-MDR approval, Lunit is now able to offer INSIGHT CXR4 to healthcare institutions across Europe. The certification confirms compliance with the EU’s strengthened requirements for safety, performance, and clinical validation.
“CXR4 goes beyond detection—it's designed to solve practical workflow issues for radiologists,” said Brandon Suh, CEO of Lunit. “With features like normal flagging and longitudinal comparison, it helps clinicians prioritize reading and improve diagnostic throughput. CE-MDR approval is an important step toward broader adoption.”
The company is now preparing additional regulatory submissions to expand availability in other global markets and accelerate integration of its AI suite into diverse healthcare systems.