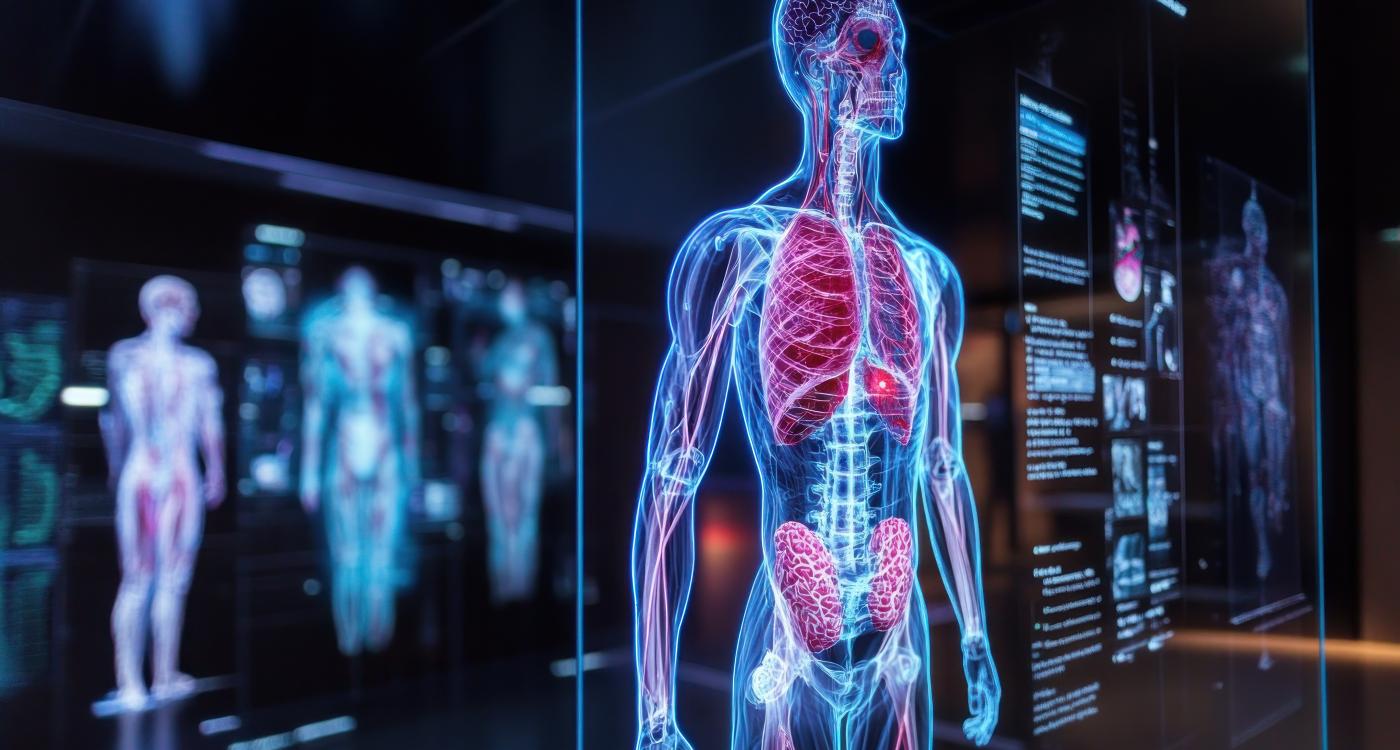
Bayer has officially launched Centafore™, its new Imaging Core Lab brand, aimed at supporting imaging in clinical trials and Software as a Medical Device (SaMD) development. Building on more than 25 years of experience and over 200 supported clinical studies within Bayer, Centafore™ now enters the market as an independent Imaging Contract Research Organization (iCRO), offering its services to external partners in pharma, biotech, medtech, and digital health.
The launch will be showcased at the 2025 Annual Meeting of the American Society of Clinical Oncology (ASCO) in Chicago from May 30 to June 3 (Booth #24152).
Centafore™ provides specialized services across the entire imaging study cycle—from concept design to image acquisition, management, interpretation, and regulatory support. Its global network of imaging experts, including board-certified radiologists, enables customers to execute complex imaging protocols in over 50 countries worldwide.
The offering spans multiple therapeutic areas including:
Oncology
Cardiovascular
Central nervous system (CNS)
Dermatology
Women’s health
Pediatrics
Digital health
Centafore™ supports trials from early-stage research to Phase IV as well as SaMD development, all while ensuring compliance with regulatory and data privacy standards.
First previewed at ECR 2024, Centafore™ has already entered collaborations with organizations such as OBIO® and Luxsonic Technologies, further embedding imaging into data-driven drug and software development strategies.