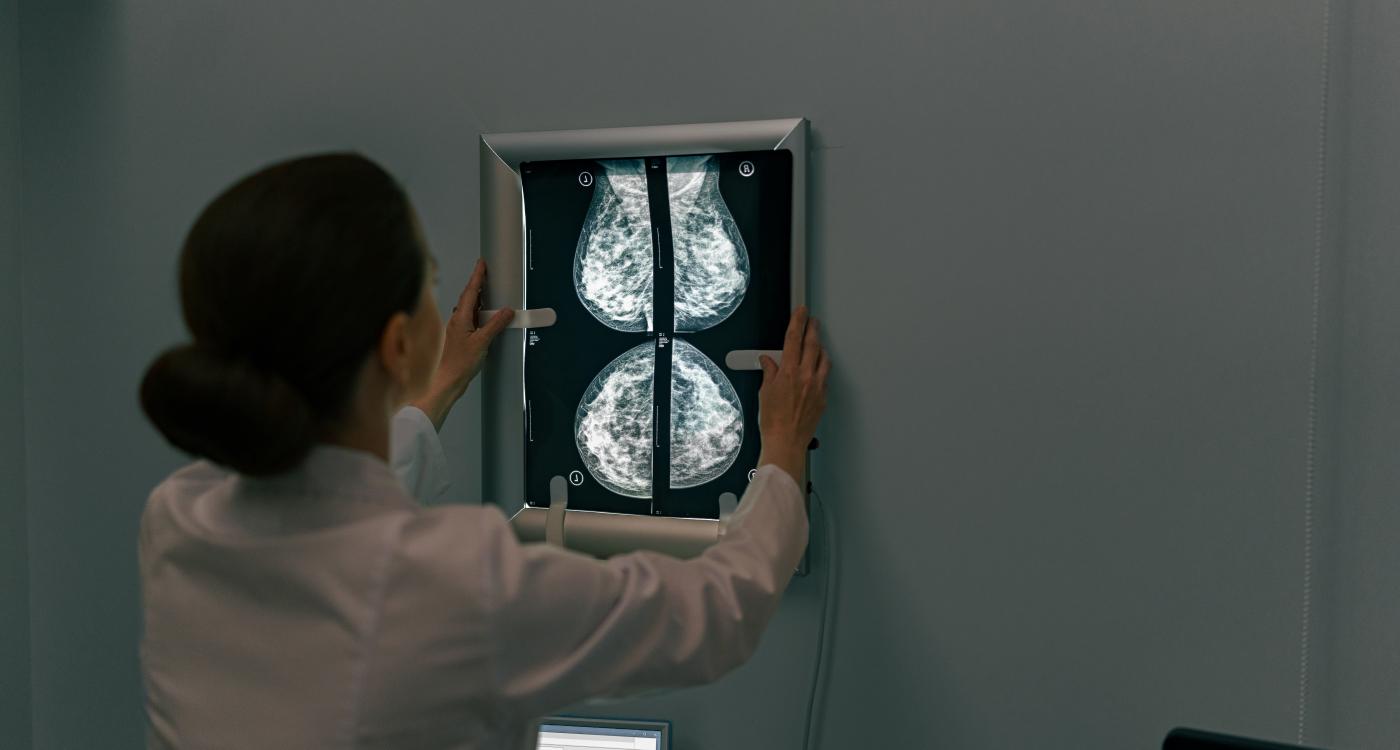
The device combines X-ray diffraction and artificial intelligence to assess molecular-level breast tissue signals and is being studied for its potential to improve diagnostic precision.
X-Ray Diffraction and AI in Breast Imaging
Calidar, Inc., a diagnostic imaging start-up from Duke University, reported the first in-human imaging with its 4D Mammography system. The investigational device applies X-ray diffraction combined with artificial intelligence (AI) to analyze the molecular composition of breast tissue. Unlike conventional mammography, which relies on shape and density, the system measures structural signatures at the molecular level, potentially improving the accuracy of cancer detection.
Clinical and Economic Burden of Current Diagnostics
Breast cancer and other soft tissue diseases remain challenging to detect with precision. Each year in the United States:
- Approximately 1.5 million breast biopsies are performed
- Up to 80% (1.2 million) prove benign
- These procedures contribute to more than $6 billion in annual healthcare costs
- An estimated 50,000 patients experience delayed diagnoses due to inconclusive imaging, which impacts treatment costs and survival rates
At the same time, radiologists and pathologists face growing workload pressures, with case volumes increasing sharply over the past decade.
How the 4D Mammography System Works
The 4D Mammography system, designed by Stefan Stryker, Josh Carpenter, and Mitchell Greene, uses X-ray diffraction imaging to produce a unique structural signature that reflects the internal composition of breast tissue. X-ray diffraction imaging reveals what the tissue is made of, not just what it looks like. In prior ex vivo studies, the technique classified benign and malignant samples with four times the precision of conventional methods.
“This is more than a study milestone — this is the start of a new era of medical imaging,” said Dr. Stefan Stryker,CEO of Calidar. “X-ray diffraction has unlocked some of the most iconic achievements in science — from discovering the structure of DNA to revealing the composition of another world on the Mars rover — and now we are bringing its power into the clinic to look inside the human body in a completely new way. Our 4D Mammography system brings this capability to the challenge of breast cancer diagnostics, where high-precision, noninvasive imaging tools are urgently needed.”
Study Collaboration and Clinical Goals
The ongoing first-in-human trial is being conducted in collaboration with Baptist Health Hardin, Elizabethtown, Kentucky, under the leadership of Principal Investigator Craig Kamen, MD. The study will compare the system’s diagnostic performance against conventional mammography devices in patients already requiring further evaluation.
“We are excited to collaborate on this next-generation research and contribute to the development of technology that could meaningfully enhance our capabilities for diagnosing breast cancer,” said Dr. Kamen.
Regulatory Status
The 4D Mammography system is investigational and has not been cleared or approved by the U.S. Food and Drug Administration (FDA). It is currently available only for investigational use in the U.S. under FDA’s abbreviated IDE requirements.
Source: Calidar, Inc