- 1.5 Tesla system does not require a quench pipe and can be sited close to the linear accelerators to reduce time between imaging and treatment
- Benefits of MRI in radiation therapy include soft-tissue contrast and functional information for tumours and organs at risk
- The use of a dedicated MRI system can enable adaptive radiation therapy through more efficient workflows and new applications
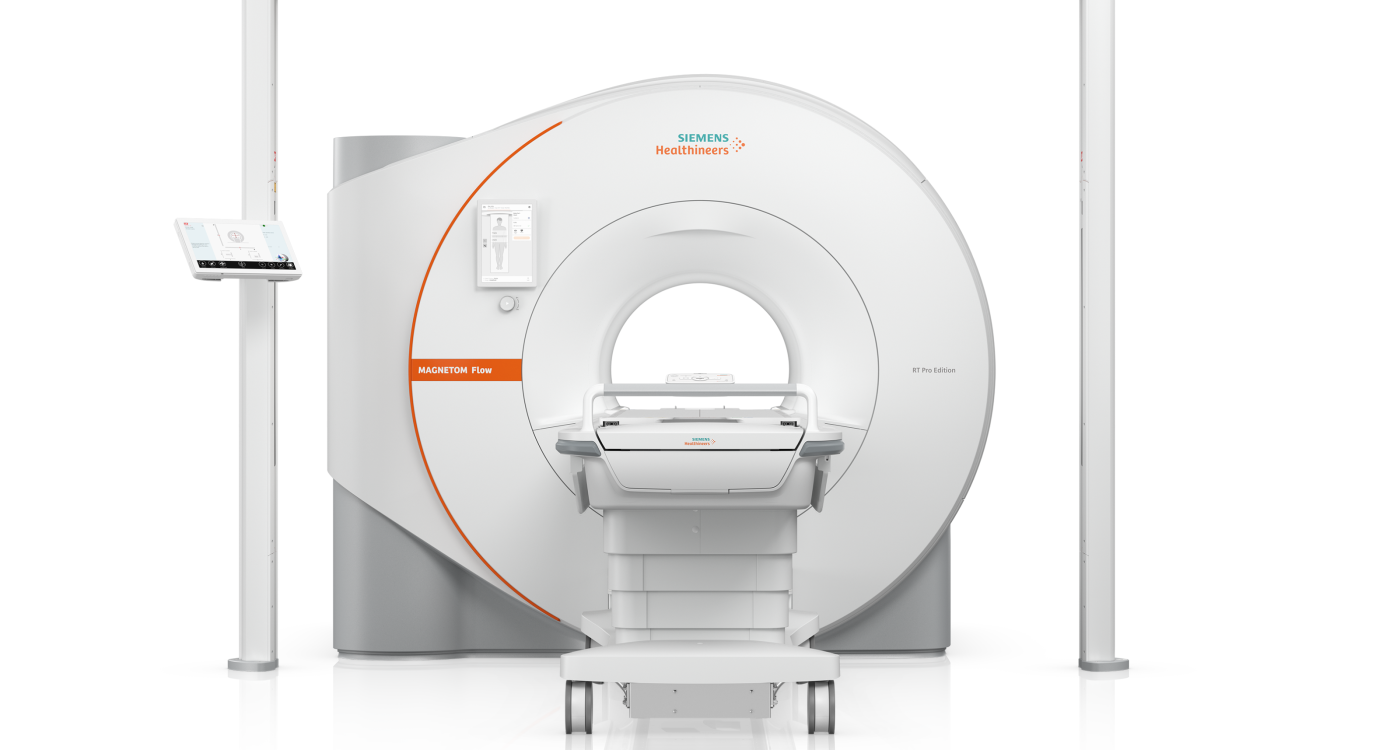
Siemens Healthineers showcased its latest MRI system for radiation therapy at this year’s European Society for Radiotherapy and Oncology congress. The 1.5T (Tesla) MAGNETOM Flow RT Pro Edition is helium-independent and does not require a quench pipe – this means it can be sited close to the linear accelerators. Through the artificial intelligence-powered image reconstruction Deep Resolve, MAGNETOM Flow RT Pro Edition delivers high-quality images while speeding up scan times. The new system is equipped with DryCool technology, meaning that it only requires 0.7 litres of liquid helium for magnet cooling, without the need to refill, instead of around 1,000 litres with conventional scanners. The new features Smart System Timer and Eco Power Mode enable energy savings of up to 45 per cent over previous scanners.(1)
MRI offers excellent soft-tissue contrast, enabling precise contouring, as well as functional information such as cell density enabling treatment response assessment. This can enhance the way clinicians monitor and adapt treatment for individual patients – all of which is highly relevant in radiation therapy. However, the use of MRI in radiation therapy has not been without its challenges in the past. Conventional MRI systems are large and require a quench pipe to safely allow helium to escape from the building directly into the atmosphere in the event of an emergency shutdown. Installing an MRI in the radiation therapy department and next to the linacs was complex as the thick cement walls of the linac bunkers had to be altered to accommodate the quench pipe.
To overcome this challenge and allow for easy access to MRI in radiation therapy, Siemens Healthineers has developed MAGNETOM Flow RT Pro Edition. Thanks to its helium-independent technology, no need for a quench pipe and a footprint of only 25m squared, the scanner can be easily installed and operated close to the bunker that houses the linear accelerator. This saves time and makes MRI workflows in radiation therapy easier. Another benefit is that patients can be scanned in the MRI system in the treatment position. Furthermore, MAGNETOM Flow RT Pro Edition offers MR-based Synthetic CT, thereby avoiding image registration errors as well as patient scheduling challenges that occur when integrating two modalities. The new scanner can also serve as an enabler for adaptive radiotherapy, where treatment plans can be adapted as needed to achieve the right balance between targeting the treatment area and preserving healthy tissue.
Alistair Piggot, business head of magnetic resonance imaging at Siemens Healthineers Great Britain and Ireland, states: "Imaging is the foundation of radiation therapy. While CT remains the basis for treatment planning, the clinical value that MRI adds has a positive impact on patient outcomes. We are very proud to be expanding our portfolio with this dedicated 1.5T MR-Scanner, which is tailor-made to the needs of radiation therapy, easy to use and efficient, powered by AI."
(1) Energy savings results were achieved by Siemens Healthineers using both standard and optional features. There can be no ‘typical’ hospital setting (case mix, system type, etc.) and so results by users may vary with no guarantee that the same results can be achieved.
MAGNETOM Flow is a 1.5T MRI platform with 70 cm bore size systems. The systems are currently under development and not commercially available. They are not for sale in the U.S. Their future availability cannot be guaranteed.
The information shown herein refers to products of third-party manufacturers and thus is in their regulatory responsibility. Please contact the third-party manufacturer for further information.