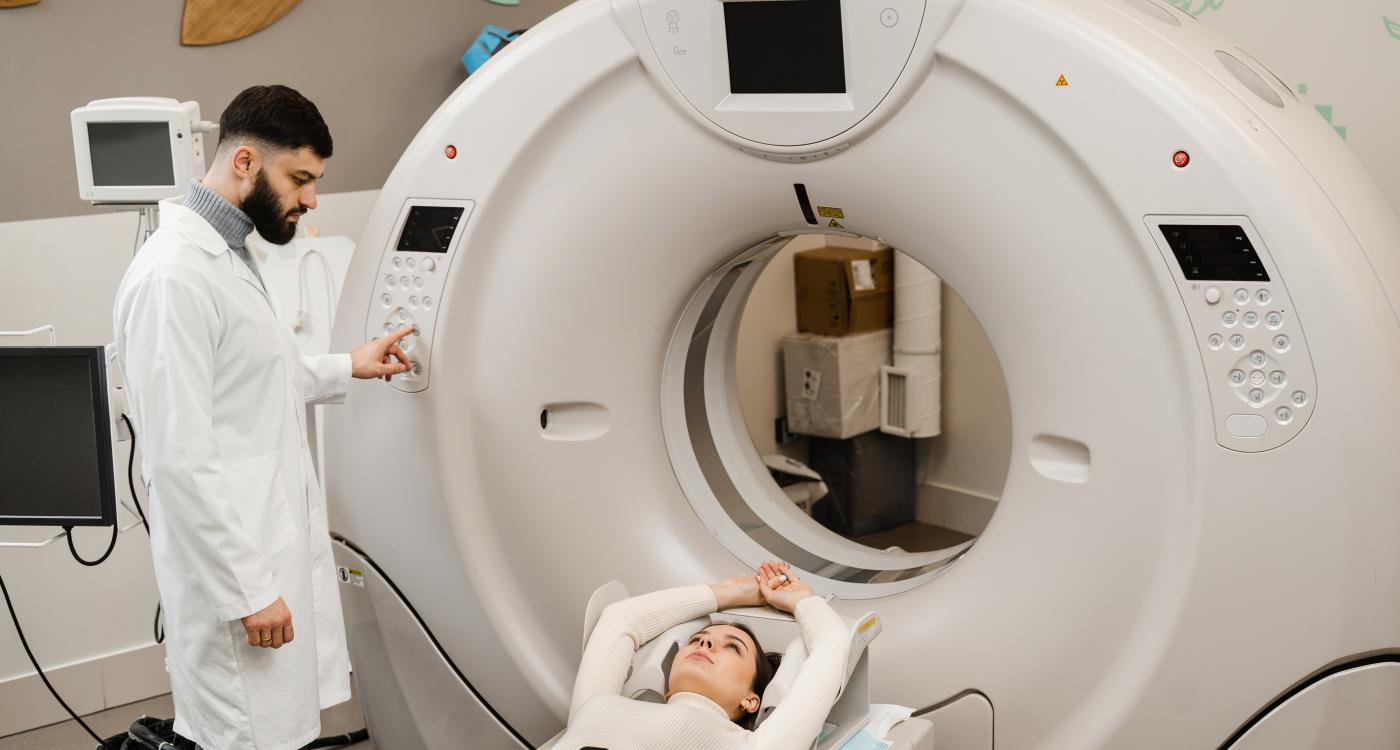
The certification covers extremity as well as head and neck imaging, supporting point-of-care 3D diagnostics in orthopedic and related clinical settings.
CE MDR Certification Expands Planmed XFI Availability in Europe
Planmed Oy has announced that its Planmed XFI® imaging system has obtained CE marking under the European Union Medical Device Regulation (MDR 2017/745). The certification covers imaging of both upper and lower extremities, as well as head and neck applications.
According to the company, the CE MDR milestone supports the availability of Planmed XFI in the European market and reflects compliance with current EU requirements for medical devices. Planmed describes the scanner as part of its cone-beam computed tomography (CBCT) portfolio, designed for orthopedic imaging and diagnostic assessment at the point of care.
Planmed stated that the system is intended to provide fast 3D imaging in clinical workflows, particularly in cases where subtle fractures or misalignments may be difficult to detect using conventional 2D radiography alone.
Weight-Bearing CBCT for Point-of-Care Imaging
Planmed XFI is positioned as a weight-bearing CBCT solution that can support imaging when anatomical structures are under load. The company highlighted that such imaging can help identify conditions that may not be apparent when the body is at rest.
The scanner has been designed for both preoperative and postoperative imaging, offering ultra-high resolution and adaptability for different patient needs. Planmed also noted its focus on detecting fractures and alignment issues during the first clinical visit.
CBCT-based imaging can provide additional diagnostic insight compared with standard radiographic approaches, particularly for musculoskeletal assessment.
Company Statements on European Market Expansion
Planmed Managing Director Jan Moed commented on the certification and broader availability of the system:
“We are excited that this innovative, low dose orthopedic imaging system is now available both in the U.S. and in Europe. With the experience that we have from the USA, we are confident that it will be well-received in Europe as well,” states Jan Moed, Managing Director of Planmed Oy.
Moed also emphasized customer demand in the region: “We are very pleased to expand our CT imaging equipment line. Many of our customers in Europe have been looking forward to this certification,” continues Moed.
Planmed added that its imaging solutions are known for user ergonomics, design, and image quality, and that the MDR certification marks an important step for clinical adoption across European healthcare environments.
Presentation Planned at ECR 2026 in Vienna
Planmed confirmed that Planmed XFI will be presented to the radiology community during the European Congress of Radiology (ECR) in Vienna, taking place March 4-8, 2026, providing an opportunity for imaging professionals to view the system in person.
Source: Planmed Oy